10 Terms
10 TermsHome > Terms > English, UK (UE) > Boyle's law
Boyle's law
States that when the temperature is held constant, the volume of a gas is inversely proportional to its pressure. Therefore, if the pressure increases, the volume decreases and visa versa. For example, if the volume if halved, then the pressure is doubled. If the temperature is held constant, it becomes an isothermal process. Discovered by Robert Boyle (1627-1691), an Irish physicist and chemist and co-founder of the Royal Society.
This is auto-generated content. You can help to improve it.
0
0
Improve it
- Part of Speech: noun
- Synonym(s):
- Blossary:
- Industry/Domain: Weather
- Category: General weather
- Company:
- Product:
- Acronym-Abbreviation:
Other Languages:
Member comments
Terms in the News
Featured Terms
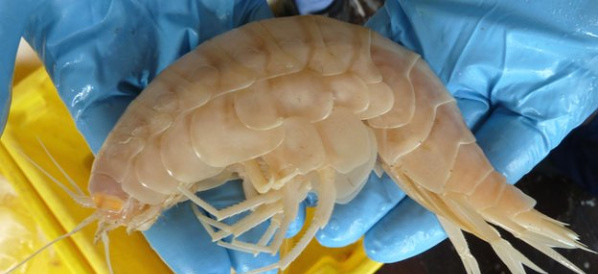
Industry/Domain: Animals Category: Crustaceans
Supergiant
A huge crustacean has been found lurking 7km down in the waters off the coast of New Zealand. The creature is called a supergiant and is a type of ...
Contributor
Featured blossaries
stanley soerianto
0
Terms
107
Blossaries
6
Followers
10 Best Value Holiday Destinations 2014
Category: Travel 5  10 Terms
10 Terms
 10 Terms
10 Terms
Browers Terms By Category
- General law(5868)
- Contracts(640)
- Patent & trademark(449)
- Legal(214)
- US law(77)
- European law(75)
Law(7373) Terms
- Satellites(455)
- Space flight(332)
- Control systems(178)
- Space shuttle(72)
Aerospace(1037) Terms
- Zoological terms(611)
- Animal verbs(25)
Zoology(636) Terms
- Conferences(3667)
- Event planning(177)
- Exhibition(1)
Convention(3845) Terms
- Organic chemistry(2762)
- Toxicology(1415)
- General chemistry(1367)
- Inorganic chemistry(1014)
- Atmospheric chemistry(558)
- Analytical chemistry(530)