21 Terms
21 TermsHome > Terms > English, UK (UE) > Helmholtz free energy
Helmholtz free energy
A thermodynamic property that can be used to predict whether a process will occur spontaneously at constant volume and temperature. Helmholtz free energy A is defined as A = U - TS where U, T and S are the internal energy , temperature , and entropy . Changes in A correspond to changes in free energy for processes occuring at constant temperature and volume. The sign of Delta A is negative for spontaneous processes and zero for processes at equilibrium .
This is auto-generated content. You can help to improve it.
0
0
Improve it
- Part of Speech: noun
- Synonym(s):
- Blossary:
- Industry/Domain: Chemistry
- Category: General chemistry
- Company:
- Product:
- Acronym-Abbreviation:
Other Languages:
Member comments
Terms in the News
Featured Terms
Industry/Domain: Network hardware Category:
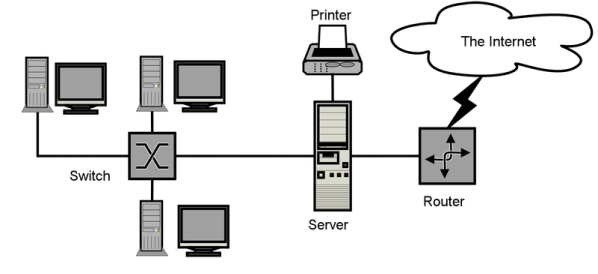
Computer network
System of interconnected computer equipment that permits the sharing for information
Contributor
Featured blossaries
Bagar
0
Terms
64
Blossaries
6
Followers
Dark Princess - Stop My Heart
Category: Entertainment 1  10 Terms
10 Terms
 10 Terms
10 Terms
Browers Terms By Category
- General Finance(7677)
- Funds(1299)
- Commodity exchange(874)
- Private equity(515)
- Accountancy(421)
- Real estate investment(192)
Financial services(11765) Terms
- Journalism(537)
- Newspaper(79)
- Investigative journalism(44)
News service(660) Terms
- Cosmetics(80)
Cosmetics & skin care(80) Terms
- Hair salons(194)
- Laundry facilities(15)
- Vetinary care(12)
- Death care products(3)
- Gyms(1)
- Portrait photography(1)
Consumer services(226) Terms
- Hand tools(59)
- Garden tools(45)
- General tools(10)
- Construction tools(2)
- Paint brush(1)