26 Terms
26 TermsHome > Terms > English, UK (UE) > Off label use
Off label use
A term used for procedures that are legal, but are not specifically approved for a particular device or drug. An example is Lasik. The excimer laser is FDA approved for PRK, but not for Lasik, which is a combination of ALK and PRK. The Lasik procedure comes under the definition of the practise of medicine (also called scope of practice), so the FDA does not necessarily need to approve the use of the excimer laser specifically for Lasik. An easier example is the scalpel. The scalpel is not specifically approved for all procedures that may require a scalpel, but if a medical doctor determines the use of this FDA approved tool is appropriate, then it is okay with the FDA. Some excimer lasers have received FDA approval specifically for Lasik, but this is more for marketing purposes than to accommodate any legal requirement.
- Part of Speech: noun
- Synonym(s):
- Blossary:
- Industry/Domain: Eyewear
- Category: Optometry; Contact lenses; Eye surgery
- Company:
- Product:
- Acronym-Abbreviation:
Other Languages:
Member comments
Terms in the News
Featured Terms
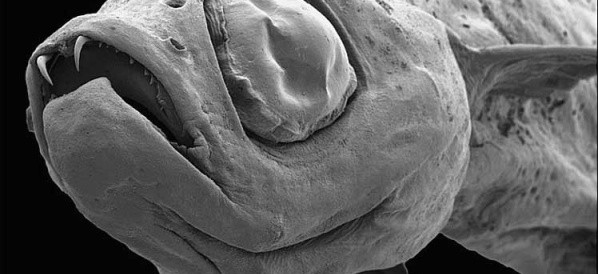
Dracula fish
A new species of fish discovered in the Mekong River region in 2009. The Dracula fish is so named because it has two fangs at the front of its top ...
Contributor
Featured blossaries
Browers Terms By Category
- Cultural anthropology(1621)
- Physical anthropology(599)
- Mythology(231)
- Applied anthropology(11)
- Archaeology(6)
- Ethnology(2)
Anthropology(2472) Terms
- Aeronautics(5992)
- Air traffic control(1257)
- Airport(1242)
- Aircraft(949)
- Aircraft maintenance(888)
- Powerplant(616)
Aviation(12294) Terms
- Railroad(457)
- Train parts(12)
- Trains(2)
Railways(471) Terms
- General astrology(655)
- Zodiac(168)
- Natal astrology(27)
Astrology(850) Terms
- Wine bottles(1)
- Soft drink bottles(1)
- Beer bottles(1)