8 Terms
8 TermsHome > Terms > English, UK (UE) > Conservation of mass principle
Conservation of mass principle
The fundamental principle used to balance chemical reaction equations. It states that the total mass of each element is conserved during a chemical reaction. The total mass of each element on the right-hand side of the reaction equation (the products) must be equal to the total mass of that element on the left-hand side (the reactants) even though the elements exist in different chemical compounds in the reactants and products. Even though the mass must be conserved, the total number of moles is not conserved during a chemical reaction.
This is auto-generated content. You can help to improve it.
0
0
Improve it
- Part of Speech: noun
- Synonym(s):
- Blossary:
- Industry/Domain: Physics
- Category: Thermodynamics
- Company:
- Product:
- Acronym-Abbreviation:
Other Languages:
Member comments
Terms in the News
Featured Terms
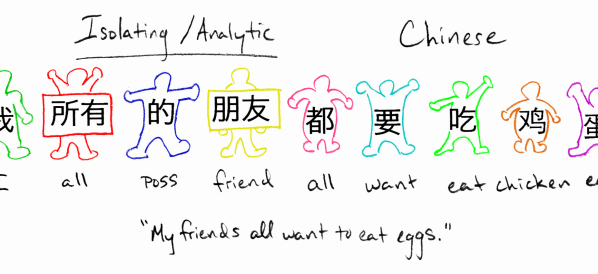
Isolating language
Isolating languages tend to form their words of single morphemes (that is, of roots without affixes). They often use several short words where another ...
Contributor
Featured blossaries
SharfuddinR
0
Terms
11
Blossaries
2
Followers
Our Planet-our environment
Category: Science 2  8 Terms
8 Terms
 8 Terms
8 Terms
Browers Terms By Category
- General boating(783)
- Sailboat(137)
- Yacht(26)
Boat(946) Terms
- Social media(480)
- Internet(195)
- Search engines(29)
- Online games(22)
- Ecommerce(21)
- SEO(8)
Online services(770) Terms
- Radiology equipment(1356)
- OBGYN equipment(397)
- Cardiac supplies(297)
- Clinical trials(199)
- Ultrasonic & optical equipment(61)
- Physical therapy equipment(42)
Medical devices(2427) Terms
- Film titles(41)
- Film studies(26)
- Filmmaking(17)
- Film types(13)
Cinema(97) Terms
- General architecture(562)
- Bridges(147)
- Castles(114)
- Landscape design(94)
- Architecture contemporaine(73)
- Skyscrapers(32)