20 Terms
20 TermsHome > Terms > English, UK (UE) > Supplement type
Supplement type
Companies are allowed to make changes to drugs or their labels after they have been approved. To change a label, market a new dosage or strength of a drug, or change the way it manufactures a drug, a company must submit a supplemental new drug application (sNDA). The supplement type refers to the kind of change that was approved by FDA. This includes changes in manufacturing, patient population, and formulation.
This is auto-generated content. You can help to improve it.
0
0
Improve it
- Part of Speech: noun
- Synonym(s):
- Blossary:
- Industry/Domain: Pharmaceutical
- Category: Drugs
- Company: U.S. FDA
- Product:
- Acronym-Abbreviation:
Other Languages:
Member comments
Terms in the News
Featured Terms
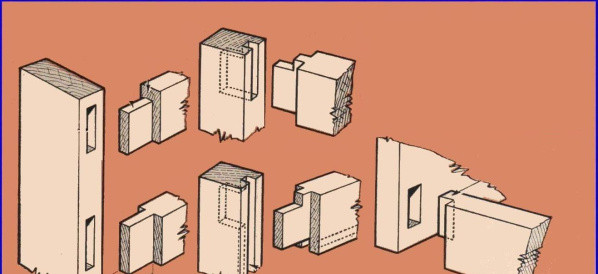
Industry/Domain: Construction Category: Windows
mortise and tenon
A strong joint wood made by the lace of a mortise in a table and one matching outgoing member (Tenon) on the other.
Contributor
Featured blossaries
Browers Terms By Category
- Christmas(52)
- Easter(33)
- Spring festival(22)
- Thanksgiving(15)
- Spanish festivals(11)
- Halloween(3)
Festivals(140) Terms
- ISO standards(4935)
- Six Sigma(581)
- Capability maturity model integration(216)
Quality management(5732) Terms
- Social media(480)
- Internet(195)
- Search engines(29)
- Online games(22)
- Ecommerce(21)
- SEO(8)
Online services(770) Terms
- Mapping science(4042)
- Soil science(1654)
- Physical oceanography(1561)
- Geology(1407)
- Seismology(488)
- Remote sensing(446)
Earth science(10026) Terms
- Algorithms & data structures(1125)
- Cryptography(11)